

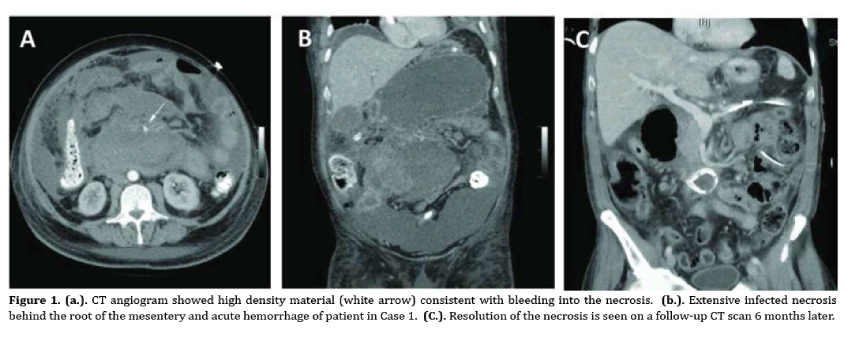
Necrotizing pancreatitis is an uncommon yet serious complication of acute pancreatitis with mortality rates reported up to 15% that reach 30% in case of infection. Traditionally open surgical debridement was the only tool in our disposal to manage this serious clinical entity. This approach is however associated with poor outcomes. Management has now shifted away from open surgical debridement to a more conservative management and minimally invasive approaches. Contemporary approach to patients with necrotizing pancreatitis and/or infectious pancreatitis is summarized in the 3Ds: Delay, Drain and Debride. Patients can be managed in the intensive care unit and any intervention should be delayed. Percutaneous drainage can be utilized first and early in the course of the disease, followed by endoscopic drainage or video assisted retroperitoneoscopic drainage if necrosectomy is deemed necessary. Open surgery is now less frequently performed and should be reserved for cases refractory to any other approach. The management of necrotizing pancreatitis therefore requires a multidisciplinary dynamic model of approach rather than being a surgical disease.
Acute pancreatitis, in most cases, represents a mild, self-limited disease but in 15%-25% severe acute pancreatitis (SAP) develops, manifested with pancreatic parenchymal and/or peri-pancreatic tissue necrosis. Pancreatic necrosis accounts for substantial additional morbidity, with mortality rates remaining as high as 10%-20% despite advances in critical care. The clinical course of SAP is divided in two phases. An early inflammatory phase that lasts the first 2 week and a late phase after the first 2 week, marked by infectious complications. Mortality rates in the event of infected necrotizing pancreatitis increase up to 30% with surgical intervention and nearly 100% in the absence of any intervention.
Historically the only tool in our disposal was laparotomy therefore early open surgical intervention for extensive pancreatic necrosis had been broadly adopted. This previously held dogma has now matured into a dynamic and multi-modal management strategy. The volume of open surgical debridement has dramatically fallen over the years as the minimally invasive techniques prove to be effective. This paper will review the current trends in intervention for the treatment of necrotizing pancreatitis and infected pancreatic necrosis.
Acute pancreatitis is most commonly caused by gallstones or alcohol, with less common etiologies including tumor, trauma, hypertriglyceridemia, medications (e.g., azathioprine, furosemide, steroids, cimetidine) and iatrogenic injuries (e.g., endoscopic retrograde cholangiopancreatography and surgery). The pathogenesis is initially caused by unregulated activation of trypsin within the pancreatic acinar cells. After activation of trypsinogen to trypsin, several enzymes such as elastase, phospholipase A2 and the complement and kinin are activated. The release of these enzymes and the resulting injury to the pancreatic parenchyma triggers an inflammatory cascade resulting in additional cytokine production, including interleukin (IL)-1, -6 and -8, as well as tumor necrosis factor α. Additionally, activation of endothelial cells enables the migration of leukocytes with release of more injury inducing enzymes. The endpoint of this cascade is a systemic inflammatory response syndrome (SIRS), characterized by loss of vascular tone, systemic vascular resistance and increased capillary permeability with third spacing of plasma volume, leading to hypotension. SIRS can produce adult respiratory distress syndrome and multiorgan dysfunction syndrome.
The natural history of necrotizing pancreatitis is variable as it may remain solid or liquefy, remain sterile or become infected over time. The first 2-4 days after the onset of acute pancreatitis are the most important when about 15%-25% of patients takes the course of a severe disease. If necrosis occurs, it is usually characterized by two phases. During the first phase, occurring the first 2 week, a systemic inflammatory response is predominant, which is often associated with multiple organ failure, especially after the first 72 h, conferring to 50% of the mortality. In the second, late phase that starts 14 days after the onset of symptoms, the systemic inflammation often regresses and infected necrosis occurs in about 30% of patients with necrotizing pancreatitis. The bacteriological analysis of the fluid reveals predominantly gut flora, as Escherichia coli, Enterococcus, Klebsiella, however, Staphylococcus aureus, and candida species have been observed.
The early management of severe acute pancreatitis and necrosis is of great importance and should take place in the intensive care unit, mainly consisting of vigorous resuscitation to overcome the substantial third spacing resulting from peripancreatic inflammation and capillary leak. Administration of antibiotics in case of pancreatic necrosis without documented infection remains a controversial area. Prophylactic antibiotics were generally recommended in the past but more recently, randomized studies have failed to show clear benefit. Although current literature does not support use of prophylaxis in all cases of severe acute pancreatitis, early empiric use in patients with clinical signs of infection (fever, leukocytosis, hemodynamic instability) is clearly advocated. Indication for surgical intervention is when there is documented or suspected infection. The presence of infection can be established with a positive computed tomography (CT) guided FNA although it is not the standard of care. Infection can be presumed with the presence of extraluminal gas in the pancreatic or peripancreatic tissues on CECT. Patients without documented infection and with clinical deterioration, SIRS, and MOFS are no longer thought to be immediate candidates for surgical intervention and surgery is reserved as the last resort. In particular, the first week of acute pancreatitis characterized by SIRS has very poor prognosis regardless surgical intervention. Emergent surgery regardless the timing is indicated in case of abdominal compartment syndrome and intestinal perforation as a result of fulminant necrotic pancreatitis. Sterile acute necrotic collection will require surgical intervention only in the presence of significant mechanical obstruction, such as biliary and gastric outlet obstruction and failure to thrive.
Available methods for intervention include the open approach, the minimally invasive approaches with percutaneous catheter placement, laparoscopic and retroperitoneoscopic approach, endoscopic and lastly hybrid approaches that will be analyzed below:
Although open surgery during the early phase can be associated with mortality rates up to 65%, randomized data confirms the benefit of late surgical intervention (at least 12 days after the onset of symptoms) with decrease of mortality to 27% and even lower between 10%-20% in specialized centers. Necrosectomy is performed either through a subcostal or a midline longitudinal incision. The retroperitoneum is entered through the lesser sac and the pancreas is exposed. In cases that the above approach is not feasible, infracolic approach has been described as alternative. Debridement is typically performed with blunt finger dissection or ring forceps representing an organ-sparing technique. Formal resection is avoided to minimize the incidence of bleeding, fistulae and removal of vital tissue. Enterotomies are avoided, again to decrease the incidence of post-operative enterocutaneous fistula. Cholecystectomy can be added to the procedure in cases of gallstone pancreatitis. The area of necrosectomy is irrigated with several litters of saline.
Two distinct open surgical completion techniques have been described: (1) open abdominal packing, with return trips to the operating room every 48 hour for further debridement until granulation tissue has replaced the retroperitoneal necrosis, a processes called "marsupialization". Some authors have described the "sandwich technique" were suction tubes were placed for superficial drainage and the wound was covered by protective materials (Opsite dressings) and a mesh was interposed between the edges of the fascia. All reoperations can be made in the surgical intensive care unit (ICU). Wounds were permitted to heal by secondary intention; and (2) continuous post-operative lavage. This technique involves insertion of two or more double lumen Salem® sump tubes and single lumen silicone rubber tubes through separate incisions with their tips in the lesser sac and necrotic areas. The smaller lumen tubes are used as the inflow and the larger lumen tubes for outflow. Thirty five to forty litters of fluid are used for lavage. Drains can be removed within 2-3 week. Alternatively, "closed packing" is similar to continuous lavage, but also involves multiple, large gauze-filled Penrose® drains that pack the abscess cavity and control minor bleedings. Drains can be removed after a minimum of 7 days.
The above techniques are associated with complications in the immediate post-operative period as well as long term. Potential immediate complications include hollow viscus perforation, organ failure, infection, wound dehiscence and end organ failure such as renal failure. Bleeding is rare and can be managed angiographically. Long-term complications include incisional hernias, gastrointestinal fistula, gastric outlet stenosis, colonic and pancreatic fistulas. All the above are more common with the open techniques. Additionally, exocrine and endocrine pancreatic insufficiency is another known long term complication. Morbidity varies between studies and rates 34%-95% have been reported. Mortality averages between 10%-20% in most studies. Between the two above-mentioned open techniques, the closed continuous lavage is most commonly used.
Laparoscopic approach for pancreatic necrosectomy is not so widely advocated and no large series or randomized studies are available. Parekh in 2006 described a laparoscopic technique utilizing 3 ports and a hand port for infra-colic approach and blunt dissection with the fingers or with an endo-dissector and several drains left in 19 patients. Indications were mainly, documented necrosis, progressive organ failure, or persistent symptoms. Success rate was 77% but mortality was 11% with morbidity rate reported 58% mainly including pancreatic fistula, central line infections and clostridium difficile infection. Advantages of this minimally invasive technique are less wound infections and risks include dissemination of retroperitoneal infection into the retroperitoneum. Specialized centers have reported laparoscopic drainage of necrotic collections, once they are walled off, either in the stomach or the small bowel, but this technique is not widely used, due to the technical challenge associated.
This approach is a modified laparoscopic approach and includes a constellation of modified techniques that utilize a percutaneous tract, usually created under CT guided drainage. This tract is dilated so that a rigid nephroscope, endoscope or even a laparoscope is advanced to provide direct visualization of the necrosis. Then an incision is made through a left translumbar approach or a small subcostal incision (5-7 cm) and debridement and lavage is performed until resolution of the necrosis. The term widely used to describe all the above is video assisted retroperitoneal debridement (VARD) and previously used terms as sinus tract endoscopy. Horvath et al, in 2010, performed a multicenter prospective study to evaluate the safety and efficacy of VARD utilized in 40 patients with infected pancreatic necrosis diagnosed by FNA. A retroperitoneal percutaneous drain was placed within 48 hours of admission and was upsized every 3-4 days until a 20 French drain was reached and that was eventually used as the VARD route. From the 40 patients initially enrolled, 25 underwent VARD and 81% required only one trip to the OR (success rate). Patients crossing over to open surgery were found to have a central collection with inferior extension to the mesenteric root, therefore not amenable to drainage or VARD through the required retroperitoneal approach. The authors reported the associated morbidity, including 6% hemorrhage, 10% enteric fistulas and no mortality. Overall in the literature an average success rate is reported as high as 88%, mortality ranges from 0%-20% and peri-procedural morbidity 10%-30%.
Endoscopic necrosectomy is widely used for infected pancreatic necrosis as a means of a minimally invasive approach. It utilizes moderate sedation (midazolam or propofol and fentanyl) and endoscopy to advance an endoscope in the stomach. Approach to the area of necrosis can be performed either through the stomach or the duodenum. Puncture of the fluid collection can be made either directly by visualizing a bulge or with endoscopic ultrasound (EUS) guidance with the latter being more technically successful and with less adverse effects. The collection is punctured with a 19-gauze needle and a guide-wire is advanced under fluoroscopic guidance. The tract is balloon dilated up to 8mm. Then, 2 or more double pigtail plastic stents are placed and the collection is irrigated with 1 liter of normal saline per 24 h. Necrotic tissue is evacuated with a basket, a net or a polypectomy snare. A systematic review of endoscopic necrosectomy of pancreatic necrosis by Haghshenasskashani et al in 2011 revealed an overall 76% definitive resolution with endoscopic techniques alone, with a median of 4 sessions. Mortality was 5% and morbidity about 30%, with most common bleeding. Fatal air embolism has been reported in a multicenter study of transluminal endoscopic necrosectomy in Germany (the GEPARD study) and therefore carbon dioxide is now more commonly used for insufflation rather than air. Additionally, this study reports success rate of 80% after a mean of 6 sessions with mortality 7.5% and morbidity 26%. Bakker et al in 2012 published a randomized trial comparing endoscopic transgastric necrosectomy vs surgical necrosectomy for infected necrotizing pancreatitis. Twenty-two patients were randomized in the study, twelve in the surgical arm and ten in the endoscopic. The endoscopic necrosectomy reduced the post-procedural inflammation as measured by the IL-6 levels, especially the first 24 h. This was also reflected in the significantly lower new-onset multi-organ failure (0% vs 50%) and pancreatic fistulas (10% vs 70%). Additionally, mortality and major morbidity was reduced in the endoscopic group when compared to the open (20% vs 80%).
More recently, "step-up" approaches in managing infected pancreatic necrosis are gaining more popularity. This approach utilizes a percutaneous drain or endoscopy to mitigate sepsis. If drainage fails to control sepsis, the next step is minimally invasive retroperitoneal necrosectomy, VARD or sinus tract endoscopy. The rationale behind this approach is the aim to control the sepsis, rather than complete removal of the infected necrosis. This reduces the rate of complications and death my minimizing the surgical trauma and the inflammatory response to a surgical intervention in already critically ill patients. This approach also addresses the challenge of intervening early in the course of necrotizing pancreatitis (first week) that is associated with increased mortality. Percutaneous drains to control sepsis are used, instead of open necrosectomy. The PANTER study, published by van Santvoort et al and Besselink et al in 2010, established a paradigm shift in managing infected necrotic pancreatitis with a conservative and minimally invasive approach. Eighty-eight patients were enrolled in the study, 44 underwent an open necrosectomy and 1 underwent VARD. In the step-up arm 43 patients were assigned to undergo minimally invasive approach according to a protocol. Specifically, the majority would begin with percutaneous drainage. If after 72 hours of observation there were no documented clinical improvement, a second procedure would be pursued, commonly endoscopic drainage and/or VARD. The 35% of the patients assigned to the step-up approach, were treated with percutaneous drainage only. New onset multi-organ failure occurred less in the minimally invasive step-up approach group compared to the open necrosectomy (12% vs 40%). Although mortality was not significantly different between the two groups, long-term morbidity including new onset diabetes mellitus (16% vs 38%), incisional hernias (7% vs 24%) and pancreatic enzyme use (7% vs 33%) was higher in the open group and reached statistically significant difference in every parameter assessed. The same study group in 2011 published a prospective observational cohort study of 639 patients treated for pancreatic or peripancreatic necrosis. Sixty-two percent of the patients enrolled in the study were treated conservatively and 38% with an intervention (PCD, endoscopic transluminal catheter drainage, VARD, open necrosectomy). Mortality in the conservative group was 7% and 27% in the group undergoing intervention. Catheter drainage was the first intervention in 63% of cases and no additional necrosectomy was required in 35% of patients. In choosing the correct approach to the necrotizing pancreatitis, an important aspect of management is timing and a randomized study two retrospective studies and a prospective study clearly show a clinical benefit from postponing debridement for approximately 4 week after admission. The use of less invasive techniques prior to that, if needed, will allow surgical debridement to be deferred or eventually avoided if possible.
Most cases of acute pancreatitis are self-limited. However, necrotizing pancreatitis and more so infected necrosis, when they develop, can be associated with increased morbidity and mortality, making management a challenge. In the past, surgical necrosectomy through a laparotomy has been the mainstay for treatment of infected necrosis and cases of clinical deterioration despite maximal treatment. This approach is however associated with poor outcomes, is seldom used and should be considered as the last resort only. The management of infected necrosis has shifted towards less invasive approaches. It is now clearly recommended that a multidisciplinary group, when approaching a patient with severe acute pancreatitis complicated by necrosis and/or infection, should gear treatment towards the "3Ds" (Delay-Drain-Debride). Drainage early in the course of the disease, followed by endoscopic drainage, VARD or laparoscopy if debridement is necessary.
More randomized studies comparing a large number of this remarkably heterogeneous group of patients will further elucidate a more consistent protocol. The clinical features of each patient will currently dictate an individualized management plan made by experts in the appropriate setting, with the appropriate resources and equipment.
